This past week I have been leading astronomy labs about Spectroscopy. So far, this has been my favorite lab. I realized, after having taught the same lab three times by now, I’m pretty comfortable with the introductory concepts of spectroscopy – so why not write a blog post on it?!
First, what even is spectroscopy?
Spectroscopy is the study of how light interacts with matter. Your first thought may be, “Like how when light bounces off of an object?” Or maybe you remember that objects appear a certain color because all colors – except the one we see – are absorbed. Unfortunately these first impressions can be confusing. Spectroscopy studies light emitted and absorbed, in particular looking for the specific wavelengths that are indicative of certain elements.
Next, how does it work?
For this, we must begin with two bits of background knowledge; the wave-like behavior of light and the structure of an atom.
Light is a self-propagating electromagnetic wave. This is a fancy way of saying light made of an electric field that, as it changes with time, creates a magnetic field. This magnetic field then creates an electric field as it changes in time.. and so on (self propagating). Due to this, we can talk about the wavelength, amplitude, period and frequency of light as through it was a wave much like you would see on the ocean or a lake.
 The wavelength (λ) is the size of the wave; the distance from the start of one crest to the start of the next. For light this can vary from the size of a building (Radio waves) to the size of a nucleus inside an atom (gamma rays).
The wavelength (λ) is the size of the wave; the distance from the start of one crest to the start of the next. For light this can vary from the size of a building (Radio waves) to the size of a nucleus inside an atom (gamma rays).- The amplitude (a) of a wave is the ‘height’ of a wave. For light, this is directly related to the intensity. A very bright light source emits waves with very large amplitudes, while a dim source emits waves with small amplitudes.
- The period of a wave is how long it takes for a whole wave to pass by you – from one crest to the next. This is intimately related to the frequency – the number of crests that reach you in one second. [They have an inverse relationship.]
All light travels at the same speed, c. This is 3 x 10^8 meters per second, or in more familiar units, 670 million miles per hour. Light travels fast! A very important relationship for light is
c = (wavelength)(frequency)
This means that if the frequency goes up, the wavelength most decrease in order to keep the speed of light constant.
We then measure the energy of light by its frequency. High frequency waves have lots of energy and low frequency waves have only a little energy. This will be important as we talk about atoms.


Niels Bohr, a Danish physicist, first introduced his model for the atomic structure in 1913. Building off of others like Ernest Rutherford and Max Planck, his model involved a dense core, the nucleus, composed of protons and neutrons. The last component, electrons, were then left to orbit this core at specific ‘radii’ or energy levels. Electrons, if given enough energy, can move from one level to another but can not sit at an energy in between. While the model of an atom has been updated from this simplistic design, the physics principles still remain relevant.
What might give an electron enough energy to move from one level to another? You guessed it, Light! If there is a wave of light at the right energy [frequency], then the light will interact with the electron causing it to absorb the light and jump to a hig her energy state. Alternatively, an electron in a high energy state can, spontaneously [or with some probability as defined by quantum mechanics], fall back down to a lower energy state. This results in a decrease in energy for the electron. Where does that energy go? It escapes the atom in the form of light – with energy [frequency] corresponding to the energy lost by the electron.
her energy state. Alternatively, an electron in a high energy state can, spontaneously [or with some probability as defined by quantum mechanics], fall back down to a lower energy state. This results in a decrease in energy for the electron. Where does that energy go? It escapes the atom in the form of light – with energy [frequency] corresponding to the energy lost by the electron.
Now on to spectroscopy:
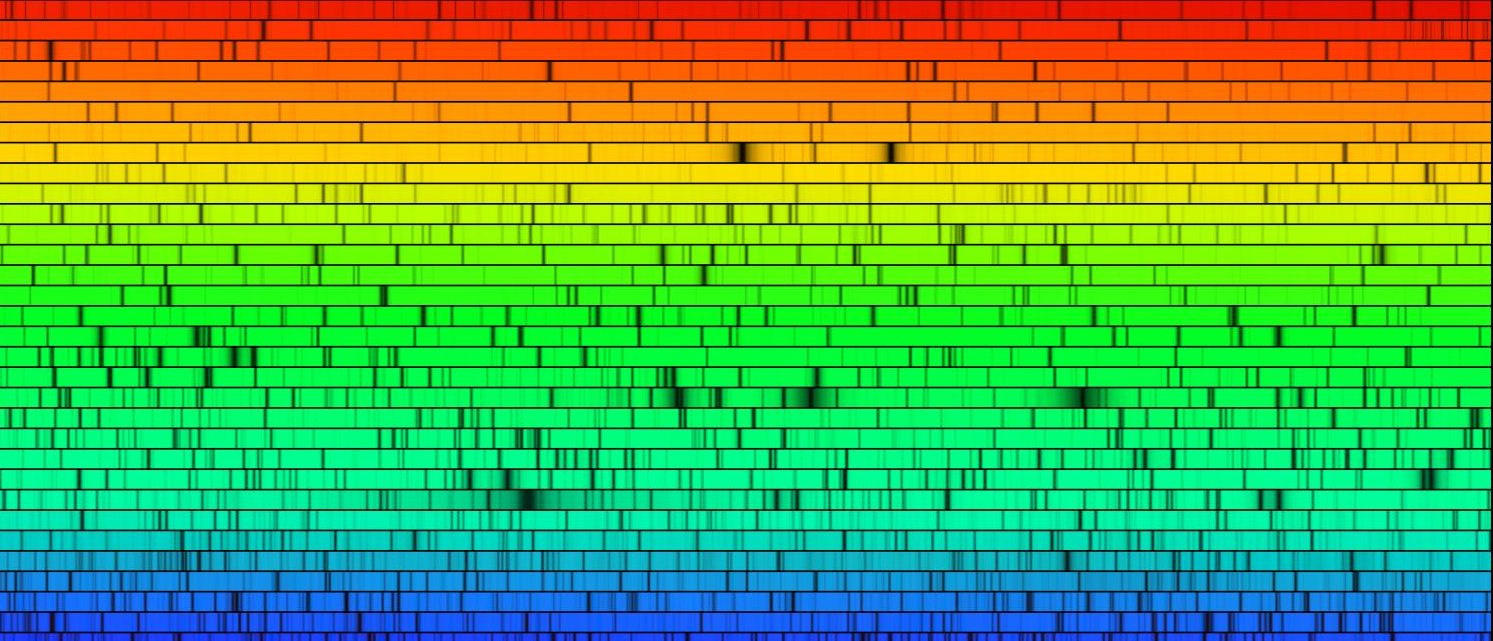
When looking at a spectra – the wavelengths of light emitted by an object – we expect three types; continuous, emission, and absorption.
A continuous spectrum comes from an object that is emitting all wavelengths (albeit with varying intensities) We call these objects blackbodies.
An emission spectrum contains only a few different wavelengths. These are created as electrons, excited by some energy source [perhaps the atoms are being warmed up by some heat source – like a star!], fall back down through their energy levels and emit specific wavelengths of light. Each element makes its own individual emission spectrum that we can use to determine what element created it.
An absorption spectrum is the inverse of an emission spectrum, it contains all wavelengths but there are some gaps [or absorption lines]. These occur when you are looking at a blackbody – emitting all wavelengths – but there is some cloud of gas in the way between the blackbody and you. This cloud of gas will absorb some of the light from the blackbody – but only the wavelengths that correspond to the correct energy to excite the electrons to a new energy level. Again, each element will absorb a specific set of wavelengths that can then indicate the type of element that did the absorbing.

Hey that’s cool! but why is it important?
Spectroscopy has many uses, though being in a household of an astrophysicist, a chemist and a cat, I mostly have astronomy, physics, and chemistry examples.
In chemistry, you often want to determine the various elements in a sample. If you take that sample and energize it (maybe you heat it up or run electricity through it) you will excite the electrons and create an emission spectrum. This can then be used to determine the type of elements in your sample.
In physics, you can analyze a blackbody spectrum and find the wavelength with the largest amplitude. This can tell you about the temperature of the object.
In astronomy, spectroscopy is key. The only way we can study things outside of our solar system is by looking at the light that comes from it. Astronomers have gotten, in my opinion, quite clever with their use of light; from developing telescopes that look in IR or UV rather than just visible light to using a spectrum from light source to determine what types of elements are in that source. Some astronomers even watch for changes in brightness of a star to determine if there is a planet nearby! (exoplanets.org)
For a star: you can look at a spectrum produced by the star learn a plethora of information. From the blackbody-like peak, you can determine the temperature of the star. The temperature can then lead you to the age of the star, and what kind of processes are happening in the core. You can also use the absorption features of the spectrum to look at what kinds of elements are in the gas-like atmosphere of the star. The elements in the atmosphere can tell you about when the star was formed, what it’s environment is like, how massive it is and how it will die.

This was a great lesson. I’d never understood much about light, and wondered what spectroscopy was and how it worked ever since I started working with doTERRA (since their oils undergo mass spectroscopy). Now I kind of get it. 🙂
Very cool; I learned stuff about waves I didn’t know before (radio: big waves, gamma: small waves) and the relationship between the size and amplitude of waves and how they must interact to keep c constant. Really enjoyed reading this!